A MedTech company providing dialysis care has raised £175 million in a Series D funding round.
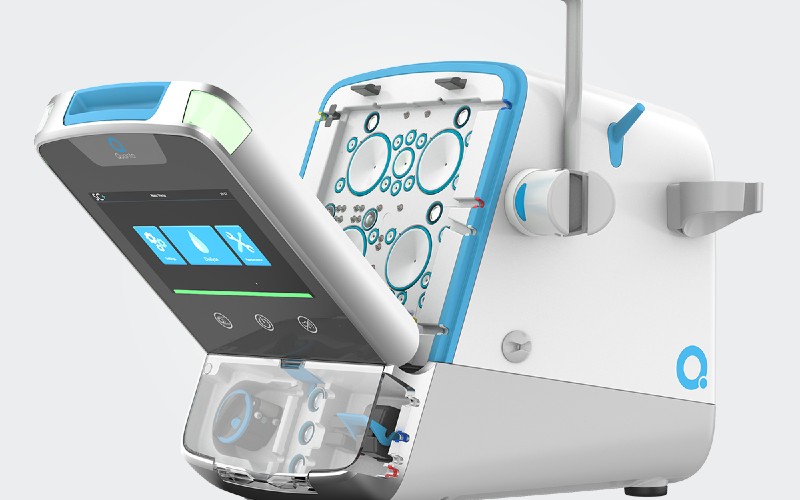
Quanta’s hemodialysis system SC+ matches the performance and dose equivalence of larger, traditional dialysis systems while also offering the portability, digital connectivity and ease-of-use of a next-generation dialysis device.
Additionally, Quanta’s companion water purification module works with SC+ as an all-in-one mobile stack, allowing dialysis treatments to be delivered in settings with just a standard water supply.
The round into the firm headquartered in Alcester, Warwickshire is the largest private funding round for a dialysis device company in history.
“We are delighted to attract such a strong syndicate of investors, which represents a clear vote of confidence in our innovative approach to dialysis treatment,” said John E. Milad, CEO of Quanta.
“Everybody knows that dialysis care must improve. For this to happen, providers and physicians need products that allow greater flexibility to bring dialysis directly to the patient, while simplifying complexity and reducing the overall cost of care.
“Our small, simple-to-use, and powerful dialysis system SC+ is positioned to help transform kidney care. The funding we are announcing today will allow us to accelerate our emergence as a significant force in this market.”
The financing round was led by Glenview Capital and co-led by Novo Holdings, with support from BlackRock, Eldridge, Sands Capital, Millennium Management, Monashee Investment Management LLC., Puhua Capital, Segulah Medical and Ancora, alongside Orlando Health, an integrated delivery network.
Existing shareholders also participated in the round, including Wellington Partners, btov, Seroba Life Sciences and The Grands. Lee Hathaway, Partner and Co-Head of Healthcare at Glenview Capital, and Robert Ghenchev, Senior Partner at Novo Holdings and Head of Novo Growth, will be joining the Quanta Board of Directors.
The funding will allow Quanta to scale up global operations with a focus on the United States, where SC+ received 510(k) clearance from the FDA in December for use in acute and chronic care facilities.
The company is investing in significant infrastructure to scale up manufacturing, sales and customer service functions to support use in currently approved care settings within the United States, while also preparing to launch a study to support its future FDA clearance for in-home use within the United States.
Lee Hathaway of Glenview Capital said: “Quanta’s innovative cartridge-based system is compact, simple, and powerful and provides tremendous value and treatment flexibility for providers and patients across an omni-channel delivery chain, from dedicated dialysis centres, acute care hospitals, and increasingly moving into the home.
“With this growth capital, Quanta has the resources to drive improved patient outcomes and convenience while accelerating market penetration and scale.”