Ultrasound tech firm ThinkSono has raised £2.1 million, achieved EU regulatory approval and secured partnerships with two US healthcare institutions as it seeks to secure FDA approval.
The round was led by id4 ventures, with participation from Brandenburg Kapital, Calm/Storm Ventures, Dubai Angel Investors, CrowdCube and Cur8 Capital as well as multiple prominent angel investors and clinicians in vascular surgery.
The investment – which takes total funding to £5m – will further propel the company’s mission to revolutionise medical imaging technology.
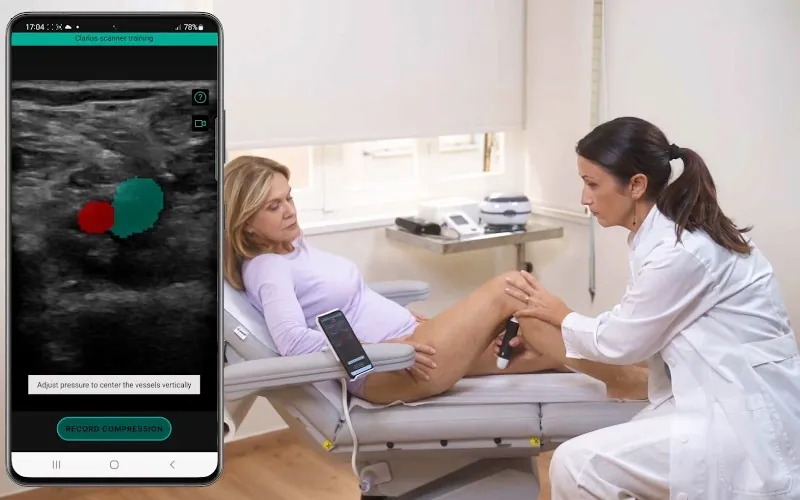
ThinkSono has attained the Class IIb CE mark for its flagship product, the ThinkSono Guidance system. This software enables non-ultrasound trained healthcare staff to scan patients with suspicion of blood clots (DVT) and send the data for qualified clinician review, thus improving the clinical pathway. DVT is a leading cause of preventable hospital death worldwide.
ThinkSono has partnered with hospitals across Europe, including the UK, Germany and Greece. It has also now secured strategic partnerships with two prestigious healthcare institutions in the US – NYU Langone Health and Temple Health – as part of its pursuit of FDA approval.
“We are thrilled to announce this significant funding milestone and the achievement of key regulatory milestones for our ThinkSono Guidance system,” said Fouad Al Noor, CEO and co-founder.
“This funding will enable us to further advance our mission of revolutionising medical imaging technology while our partnerships with NYU Langone Health and Temple Health underscore our commitment to delivering innovative solutions that ultimately improve patient outcomes.”