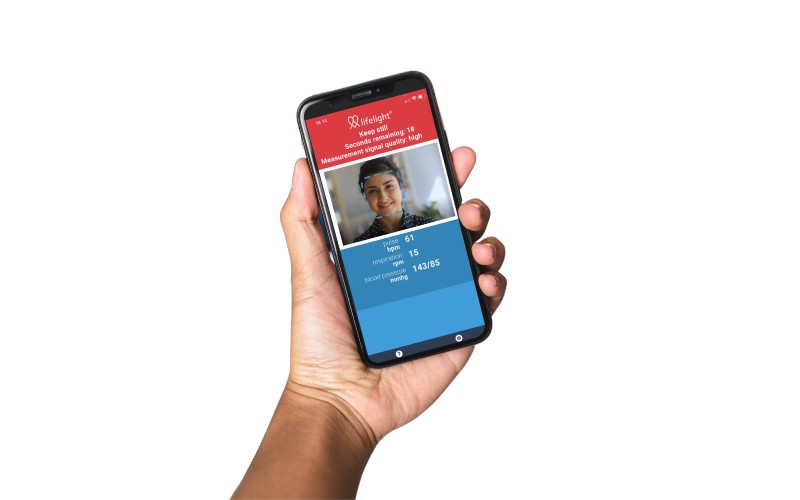
A startup has raised £6 million to power its quest to turn every device on the planet into a self-monitoring healthcare platform.
Based in Southampton, Lifelight has been supported by NHS England from inception and backed by key healthcare and industry stakeholders via grants and equity funding.
This includes a $1.5m grant from SBRI Healthcare, a prestigious NIHR & NHSX AI in Health Award, and multiple grants and loans from Innovate UK.
Accelerated by COVID, Lifelight is allowing clinicians to know a patient’s vital signs remotely, allowing accurate vitals measurements to continue to be taken in real-time with zero contact – critical during a pandemic.
It removes the cost and complexity of hardware blood pressure cuffs and pulse oximeters and can be embedded into existing healthcare platforms and pathways.
“The next year will be exciting for Lifelight. Our ethos of delivering solutions to market that adhere to clinical regulation, proven by validation, makes Lifelight a unique solution not just across Europe but worldwide,” said CEO and founder Laurence Pearce.
“Currently a CE Class I medical device, we’re working hard to deliver a CE Class II medical device and gain FDA clearance, allowing greater access to the US and beyond.”
Lifelight now employs more than 40 people and has conducted three clinical trials, while it works with telehealth organisations across multiple health sectors and geographies.
Denmark’s largest pharmaceutical company Novo Nordisk is one of those embedding Lifelight into its platforms.