Brainomix is targeting expansion in the United States of its AI platform for stroke care.
The Oxford University spinout, sixth on our MedTech 50 ranking this year, has launched its full suite of FDA-cleared modules in its Brainomix 360 platform for stroke care.
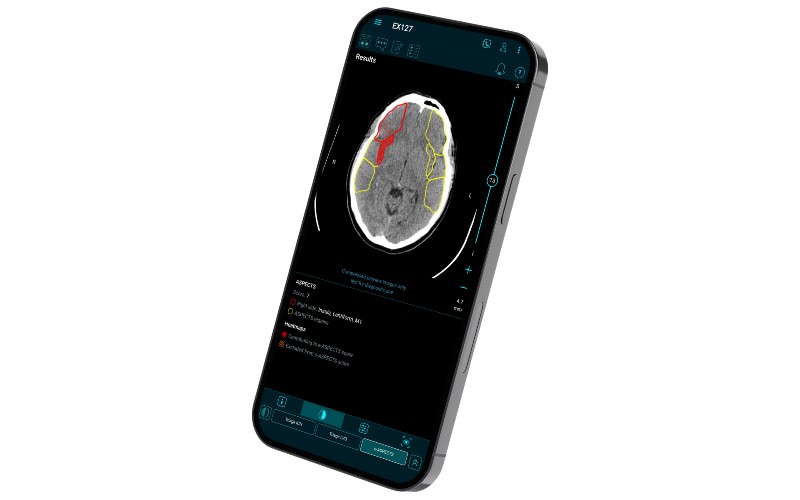
The US launch, which included its previously announced FDA-cleared e-ASPECTS module, represents a comprehensive platform designed to support clinicians and their imaging-based treatment decisions at all points across the stroke pathway, from simple imaging to more advanced imaging.
Long-established as a market leader in Europe and a pioneer in the development of innovative stroke AI solutions, the company will continue to introduce its transformative technology to more US stroke centres.
The Brainomix 360 platform is powered by AI algorithms that provide real-time interpretation of brain scans to aid treatment and transfer decisions for stroke patients, with an aim towards enabling more patients to receive the right treatment, in the right place, at the right time.
The launch included Brainomix exhibiting at the Society of Vascular and Interventional Neurology (SVIN) Conference in Miami, with Dr Waleed Brinjikji, Professor of Radiology and Neurosurgery at the Mayo Clinic in Rochester, Minnesota, providing a keynote presentation on his experience with the Brainomix 360 platform.
https://businesscloud.co.uk/medtech-50-uks-most-innovative-medical-technology-creators/
“We have been collaborating with the Brainomix team around numerous research projects over the past couple of years, including a recent study that validated the performance of their e-ASPECTS module,” noted Dr Brinjikji.
“The results showed that the accuracy of ASPECTS scoring by physicians improved across disciplines and levels of experience, which makes the e-ASPECTS module a powerful tool for clinicians across the US who are managing stroke patients.”
The recent FDA clearances included Brainomix 360 e-CTP and Brainomix 360 e-MRI, both software modules that can support thrombolysis and thrombectomy treatment decisions, particularly for late-window patients who present to hospital more than 6-12 hours after stroke onset.
Brainomix 360 Triage LVO and Brainomix 360 Triage ICH are two new notification tools, which send real-time alerts to clinicians when a bleed or large vessel occlusion (LVO) is suspected. The presence of LVO can be a key determinant when deciding a patient’s eligibility for mechanical thrombectomy.
Brainomix has established commercial operations in the US and will continue to expand as it rolls out its products across US hospital networks.
“We are delighted to have the opportunity to introduce our Brainomix 360 platform to more and more US stroke networks, and to showcase the extensive validation of our technology, a good portion of which was conducted in the US at such institutions as the Mayo Clinic, Emory University, Mount Sinai in New York, and UCLA,” said Dr Michalis Papadakis, co-founder and CEO of Brainomix.
With deployments across more than 30 countries, Brainomix’s AI stroke software has been studied and validated in more than 60 publications.